Abstract
Background:
Chronic graft-versus-host disease (cGVHD) remains a common and potentially life-threatening complication after allogenic hematopoietic stem cell transplantation (HCT). Systemic corticosteroids are first-line therapy, however, they are often associated with inadequate responses and multiple side effects. There has been no single standard therapy for refractory cGVHD. Recently, ruxolitinib, a selective JAK1/2 inhibitor, has been suggested to have efficacy as salvage therapy in cGVHD. However, data are limited to a multi-center retrospective survey of 41 patients (Zeiser et al. Leukemia. 2015; 29(10):2062-8). Herein, we present a single-institution's experience with ruxolitinib as salvage therapy for management of refractory cGVHD.
Methods:
In this single-center, IRB-approved, retrospective analysis, the charts of 47 patients who received ruxolitinib as salvage therapy for cGVHD from March 2016 through December 2016 were reviewed. Clinical data were collected and analyzed at start of ruxolitinib treatment, 3 months-post, and 6 months-post to identify changes in prednisone dose, patient perceived improvement, physician perceived improvement, and incidence of adverse events including hospitalizations, documented infections, cytopenias, relapse, and death. In addition, we assessed for treatment failure-free survival (FFS) from the time of ruxolitinib to any of the following events: initiation of new cGVHD therapy, relapsed disease, patient death, or discontinuation of ruxolitinib.
Results:
The cohort consisted of 47 patients (median age = 51; range = 21 - 91; 21 females and 26 males) who received fully ablative (n=26) or reduced-intensity conditioning (n=18) followed by an allogeneic HCT from matched related donor (n=15), unrelated donor (n=13), or other donor sources (cord or haplo-identical donors: n=16) for their hematologic disorders. The median time from HCT to ruxolitinib initiation was 40.5 months (range: 3.8 to 267.2). Of the 47 patients in this cohort, 38 patients (80.9%) had sclerotic chronic graft versus host disease.
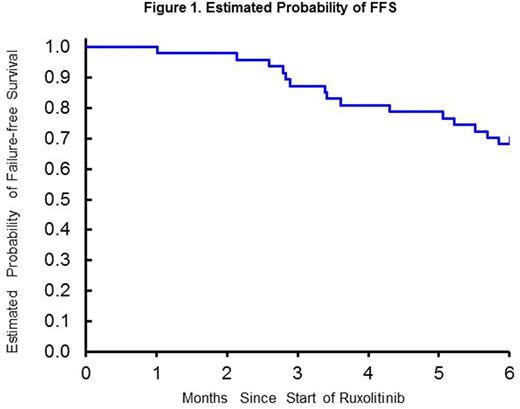
The average dose of prednisone in evaluable patients was 11.1 mg/day (95% CI 6.9 - 15.3 mg/day) at time of ruxolitinib initiation, which decreased to 7.7 mg/day (95% CI 4.1 - 11.4 mg/day, p=0.043 by paired signed rank test, 30% reduction) at 3 months, and 5.6 mg/day (95% CI 3.2 - 8.0, p<0.001, 49% reduction) after 6 months of ruxolitinib therapy. There were 12 patients (36%) and 14 patients (42%) who experienced prednisone dose reduction at 3 & 6 months, respectively. Patient-reported subjective improvement was observed in 22 of 41 evaluable patients (53.7%) and 23 of 37 evaluable patients (62.2%) at 3 and 6 months, respectively. Physicians rated subjective improvement in 29 patients (70.7%) at 3 months and 25 patients (67.6%) at 6 months. Infections were documented in 25 patients (56.8%), of which 6 (12.8%) were considered life-threatening. Two patients (4.2%) with CMV viremia with no end-organ disease were identified. Significant cytopenias requiring ruxolitinib dose reduction or discontinuation were observed in 3 patients (7.0%). During the study period of 6 months, 18 patients (40.9%) required hospitalization at least once, and 6 patients (12.8%) died within first 6 months of ruxolitinib use. Treatment failure-free survival at 6 months was 68.1% (95%CI; 52.7% to 79.4%) with the failure events including death (n=6), relapse (n=2), addition of new therapy (n=2), and discontinuation of ruxolitinib for any reason other than resolution of cGVHD symptoms (n=6) (Figure 1).
Conclusion:
Ruxolitinib is associated with prednisone reduction after 3 and 6 months, self-/physician-reported improvements, and favorable FFS at 6 months in patients with steroid-refractory chronic GVHD. Prospective randomized trials are warranted to further define its therapeutic benefits and risks of cytopenia/infectious complications.
Salhotra: Kadmon: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal